trial overview FOR PATIENTS 12+
Evaluated in 2 identical
Phase 3 trials1,2


1249 adult and adolescent patients were included in 2 identically designed double-blind, randomized, vehicle-controlled trials.1,2
In both studies, patients were randomized to monotherapy with OPZELURA, ruxolitinib cream 0.75%, or vehicle BID for 8 weeks and as needed during a 44-week extension.1,2
key inclusion criteria1,3:
- ≥12 years of age
- Diagnosis of AD ≥2 years
- IGA score of 2 or 3*
- Affected BSA of 3% to 20%
AD, atopic dermatitis; BID, twice daily; BSA, body surface area; IGA, Investigator's Global Assessment.
| TRIAL ENDPOINTS INCLUDED KEY OUTCOMES IN AD1,3 | |
| Primary endpoint | |
| Skin clearance | |
| IGA-TS | Proportion of patients who achieved clear (IGA 0) or almost clear (IGA 1) skin with at least a 2-point improvement from baseline |
| Key secondary endpoints† | |
| Itch relief | |
| Itch NRS4 | Proportion of patients with a ≥4-point improvement in itch on a 0- to 10-point scale |
| Reduction in lesion extent and severity | |
| EASI-75 | Proportion of patients who achieved ≥75% improvement from baseline in their EASI score |
| Secondary endpoints† | |
| Itch NRS change from baseline | Mean change in self-reported itch |
| EASI-90 | Proportion of patients who achieved ≥90% improvement from baseline in their EASI score |
Safety and tolerability assessments included the frequency of reported TEAEs, treatment-related AEs, and AEs leading to discontinuation.2
*Severity scale of 0 to 4.1
†This is not a comprehensive list of secondary endpoints.
AD, atopic dermatitis; AE, adverse event; EASI, Eczema Area and Severity Index; IGA, Investigator's Global Assessment; IGA-TS, Investigator’s Global Assessment treatment success; NRS, numerical rating scale; TEAE, treatment-emergent adverse event.


IN THE CLINICAL TRIALS, WHICH INCLUDED PATIENTS ≥12 YEARS OF AGE1,3:
- Patients had a mean affected BSA of ≈10%1,3
- 39% of patients had facial involvement at baseline1
- 75% of the patients had an IGA score of 3 (moderate)1
BSA, body surface area; IGA, Investigator's Global Assessment.
BASELINE PATIENT CHARACTERISTICS1
| Study Participants (N = 1249) | |
|---|---|
| Age (years) | |
| 12-17 | 20% |
| 19-64 | 71% |
| ≥65 | 9% |
| Race | |
| White | 70% |
| Black | 23% |
| Asian | 4% |
| Other | 3% |
| Lesion appearance (IGA*) | |
| Score of 2 | 25% |
| Score of 3 | 75% |
| BSA % | |
| Mean | ≈10% |
| Worst Itch Intensity (Itch NRS†) | |
| Mean score | 5 |
*Patients had a baseline IGA score of 2 or 3 on a severity scale of 0 to 4.1
†Itch NRS is defined as a 7-day average of the worst level of itch intensity in the last 24 hours, measured on a scale of 0 to 10. Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,3
BSA, body surface area; IGA, Investigator's Global Assessment; NRS, numerical rating scale.
- Patients initially randomized to OPZELURA in the TRuE-AD clinical trials remained on their regimen2
- Patients initially randomized to vehicle were rerandomized 1:1 to ruxolitinib cream 0.75% or OPZELURA2
- Patients self-evaluated active AD lesions and treated as needed (up to 20% BSA)2
- Patients were instructed to stop treatment 3 days after lesion clearance and restart treatment at the first sign of recurrence2
- If new lesions were extensive or appeared in new areas, patients were instructed to contact the investigator to determine if an unscheduled additional visit was needed. Rescue treatment was not permitted2
- Study visits occurred every 4 weeks2
- Patients initially randomized to OPZELURA in the TRuE-AD clinical trials remained on their regimen2
- Patients initially randomized to vehicle were rerandomized 1:1 to ruxolitinib cream 0.75% or OPZELURA2
- Patients self-evaluated active AD lesions and treated as needed (up to 20% BSA)2
- Patients were instructed to stop treatment 3 days after lesion clearance and restart treatment at the first sign of recurrence2
- If new lesions were extensive or appeared in new areas, patients were instructed to contact the investigator to determine if an unscheduled additional visit was needed. Rescue treatment was not permitted2
- Study visits occurred every 4 weeks2
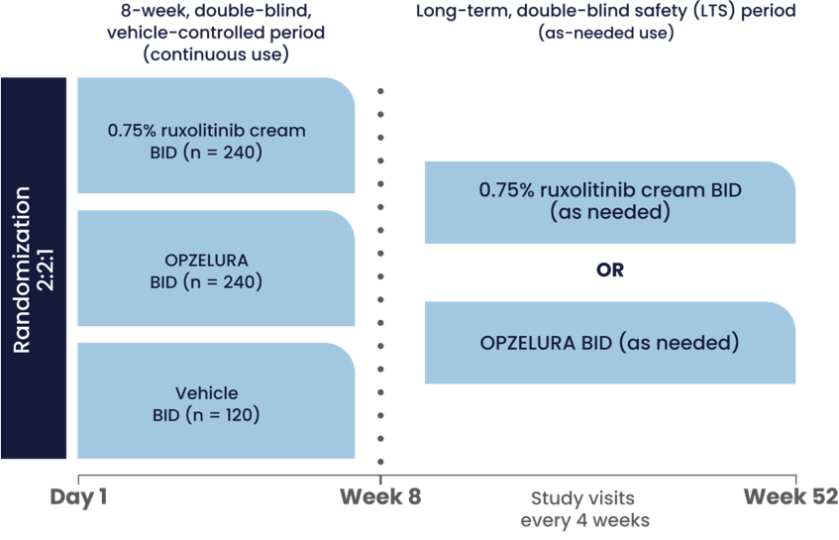

The graphic above shows the study design for OPZELURA, which was studied in 2 identically designed, double-blind, randomized, vehicle-controlled trials (TRuE-AD1 and TRuE-AD2). The 2 studies included 1249 adult and pediatric patients ≥12 years of age with an affected BSA of 3% to 20% and an IGA score of 2 (25% of patients) or 3 (75% of patients) on a severity scale of 0 to 4. Patients were randomized to monotherapy with OPZELURA, ruxolitinib cream 0.75%, or vehicle twice daily for 8 weeks.1
In a 44-week extension study, patients initially randomized to OPZELURA in the TRuE-AD clinical trials remained on their regimen. Patients initially randomized to vehicle were rerandomized 1:1 to ruxolitinib cream 0.75% or OPZELURA. Patients self-evaluated active AD lesions and treated as needed BID (up to 20% BSA). Study visits in the extension period occurred every 4 weeks.2
AD, atopic dermatitis; BID, twice daily; BSA, body surface area; IGA, Investigator's Global Assessment.



