ITCH RESULTS: AGES 12+
RAPID AND CLINICALLY MEANINGFUL ITCH RELIEF1-3
KEY SECONDARY ENDPOINT
≥4-POINT IMPROVEMENT IN ITCH (ITCH NRS4*) AT WEEK 81-3
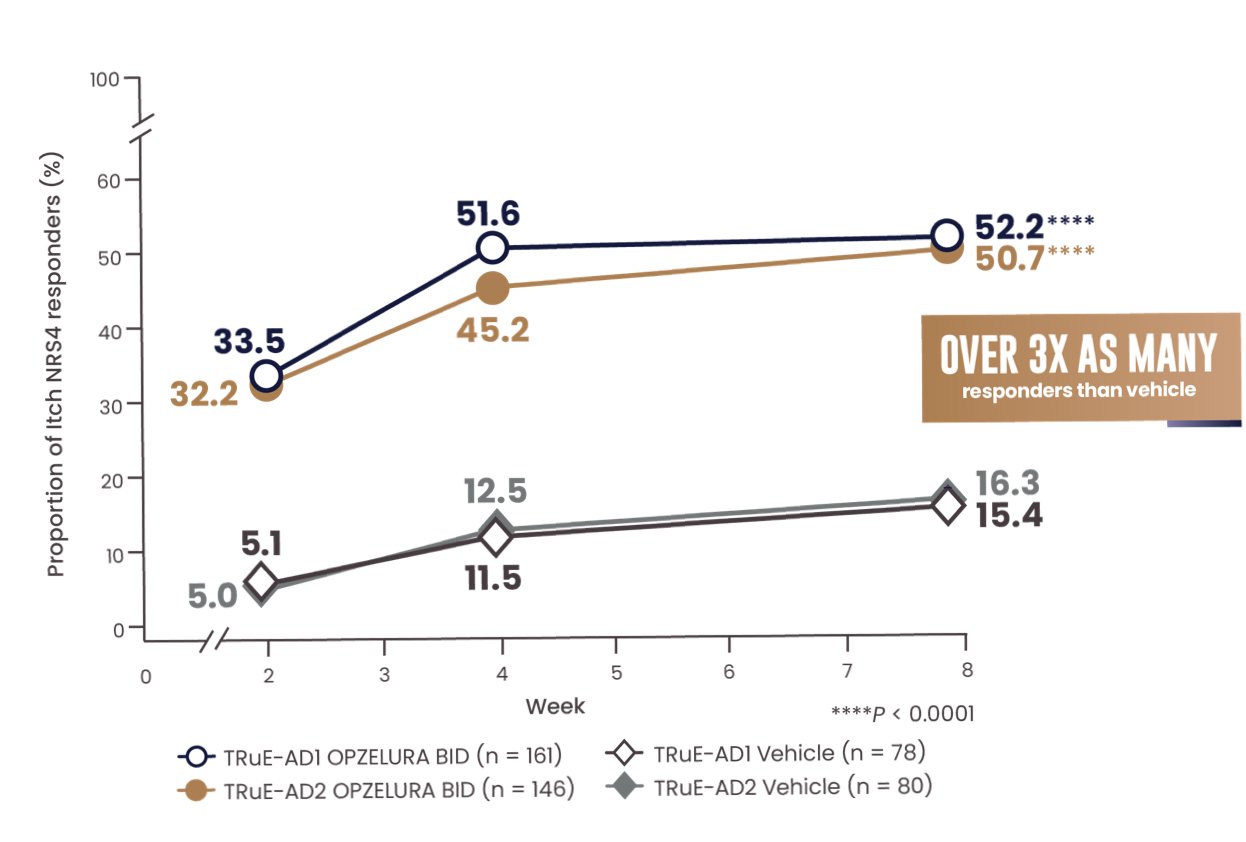

MORE THAN 50% OF PATIENTS ACHIEVED A CLINICALLY MEANINGFUL IMPROVEMENT IN ITCH (NRS4*) AT WEEK 81-3
*Itch NRS4 is defined as the achievement of at least a 4-point improvement in daily itch on a 0- to 10-point scale, considered a clinically meaningful response. Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,2
BID, twice daily; NRS, numerical rating scale.
Adapted from Papp K et al. doi:10.17632/ffx6nd5zyb.1. Licensed under CC BY 4.0
The line graph above shows the proportion of Itch NRS4 responders for OPZELURA and vehicle from the TRuE-AD1 and TRuE-AD2 clinical trials from Week 0 to Week 8. At Week 8, 52.2% of patients taking OPZELURA achieved a 4-point improvement in itch vs. 15.4% for vehicle in TRuE-AD1. In TRuE-AD2, 50.7% of patients taking OPZELURA achieved a 4-point improvement in itch vs. 16.3% for vehicle. This demonstrates over 3x as many responders to OPZELURA than vehicle.1-3
IMPACT ON ITCH
>30% of patients with Itch NRS4* at Week 2 (33.5% vs. 5.1% and 32.2% vs. 5.0%)2,3
POST-HOC, EXPLORATORY ANALYSIS
Difference in Itch NRS4 at Day 2 (NRS ≥ 4; 11.6% vs. 2.9% and 10.8% vs. 1.3%)4,5
No conclusions around efficacy should be made based on these results.
*Itch NRS4 is defined as the achievement of at least a 4-point improvement in daily itch on a 0- to 10-point scale, considered a clinically meaningful response. Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,2
NRS, numerical rating scale.
WHAT ARE PATIENTS SAYING ABOUT OPZELURA?
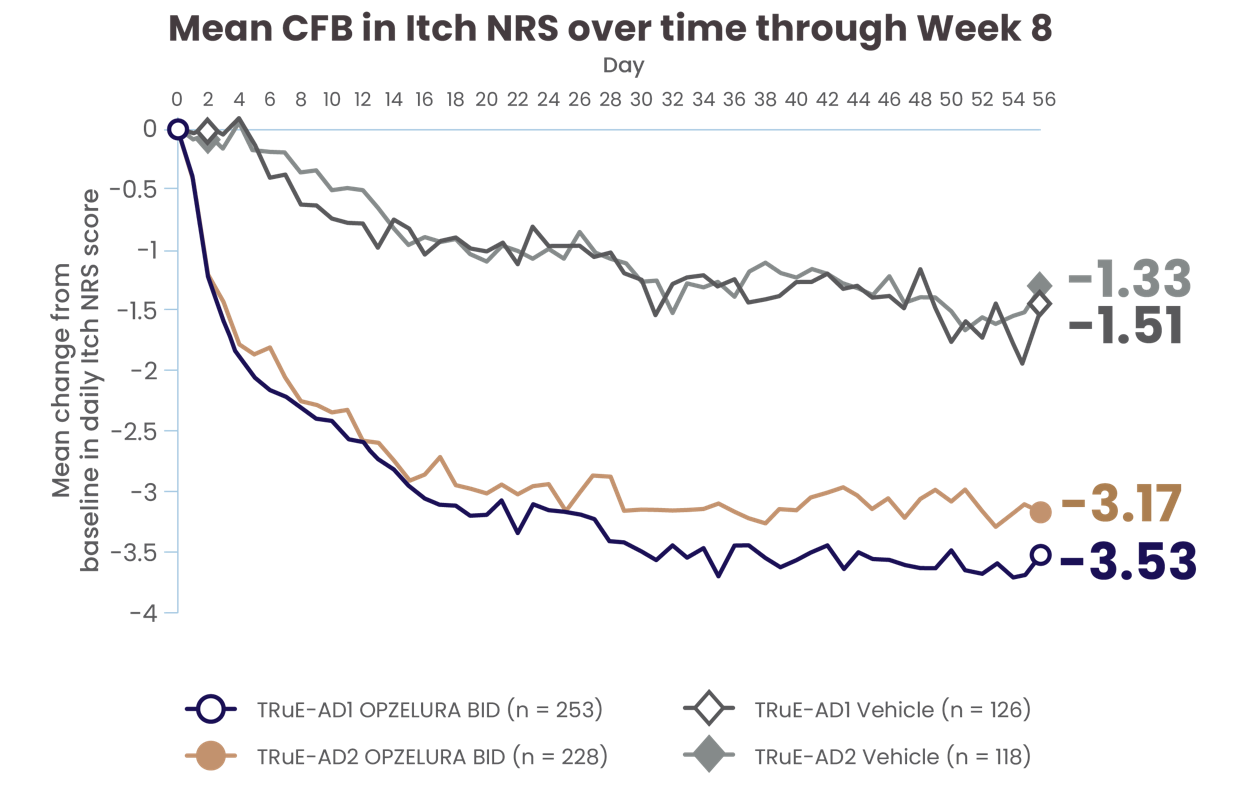
CHANGE IN ITCH NRS* THROUGH WEEK 82

ADDITIONAL EXPLORATORY ANALYSIS
PATIENT-REPORTED CHANGE IN ITCH NRS SCORE AT DAY 1† AND THROUGH DAY 562
Results were not adjusted for multiple comparisons.
Adapted with permission from Papp K et al. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
The line graph above shows the mean change from baseline in Itch NRS scores from the clinical trials from Day 0 to Day 56. In TRuE-AD1 and TRuE-AD2, the mean score at Day 56 was 3.53 and 3.17 points lower, respectively, for patients taking OPZELURA as compared with 1.51 and 1.33 points lower, respectively, for those taking the vehicle.2
*For Itch NRS assessment, patients completed an electronic diary each evening, reporting their worst level of itch during each 24-hour period from 0 (no itch) to 10 (worst imaginable itch).2
†No conclusions of efficacy should be made based on these results.
BID, twice daily; CFB, change from baseline; NRS, numerical rating scale.
ITCH NRS 0/1* MEASURED FROM DAY 1 THROUGH DAY 7—POOLED ANALYSIS6
ADDITIONAL EXPLORATORY ANALYSIS
ITCH NRS 0/1* IN THE FIRST 7 DAYS6
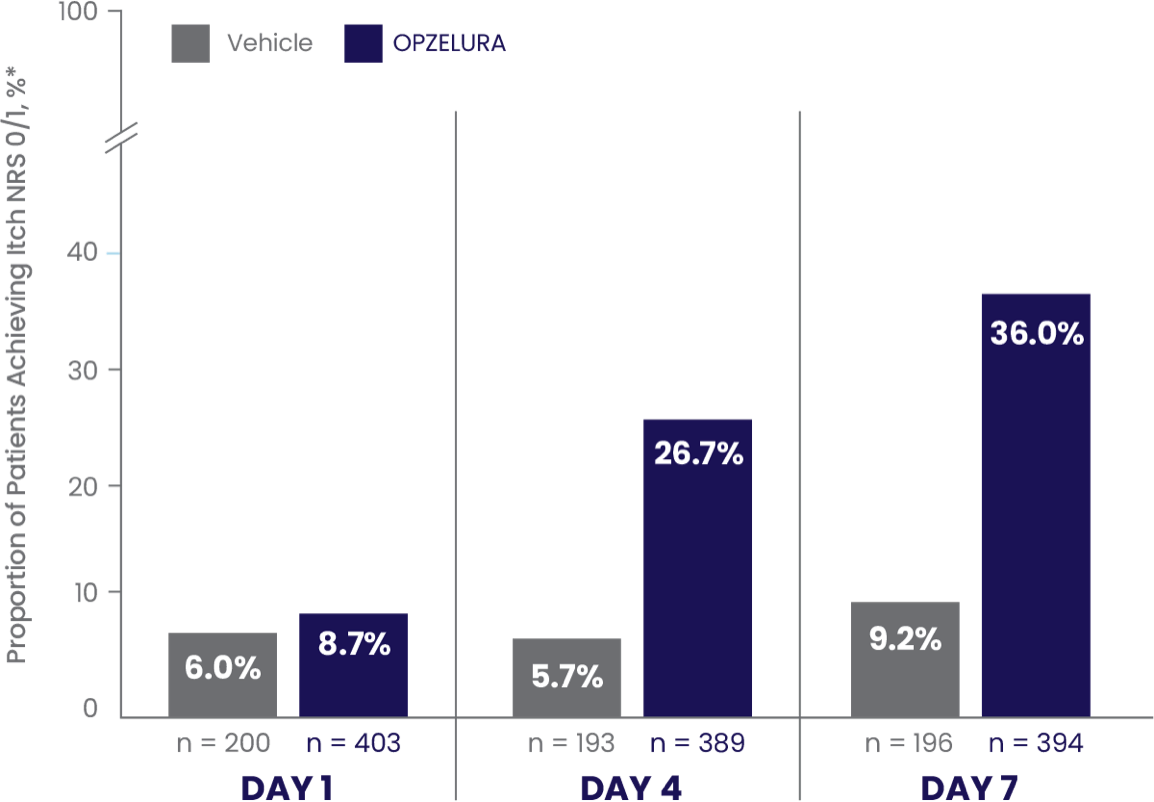
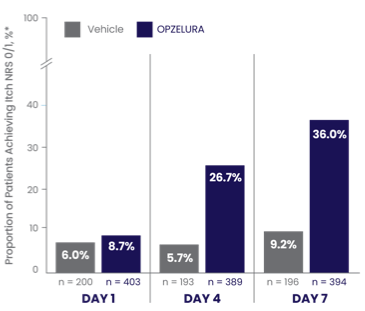
In a pooled analysis the proportion of patients who showed Itch NRS 0/1* at DAY 7 was 36.0% (and 9.2% vehicle)6
- Data were reported as observed
- No conclusions of safety or efficacy should be made based on these results
*Patients in the analysis had an Itch NRS score >1 at baseline.6
NRS, numerical rating scale.
The bar chart above shows the proportion of clinical trial participants who had Itch NRS 0/1 in the first 7 days of treatment. 36% of patients taking OPZELURA showed Itch NRS 0/1 at Day 7 vs. 9.2% for vehicle.6
SCRATCH-AD PHASE 2 STUDY7
An open-label, single-arm study of 46 patients to evaluate the effect of OPZELURA on itch in adult participants with mild to moderate AD.7
SECONDARY ENDPOINT: MEAN (SE) CHANGE FROM
BASELINE IN MPP-NRS SCORE OF 6.45,7
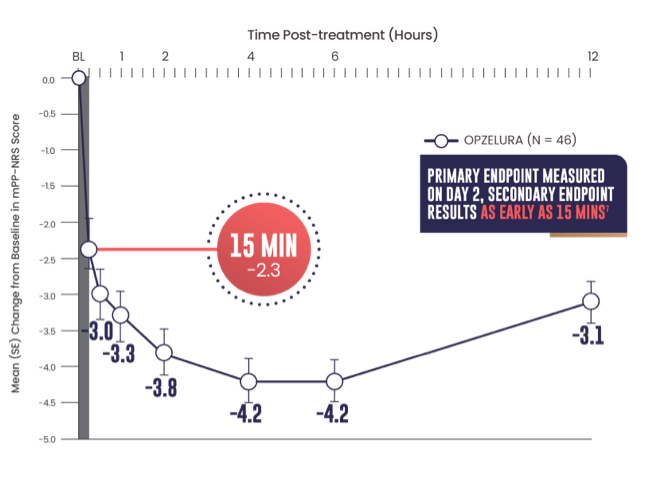
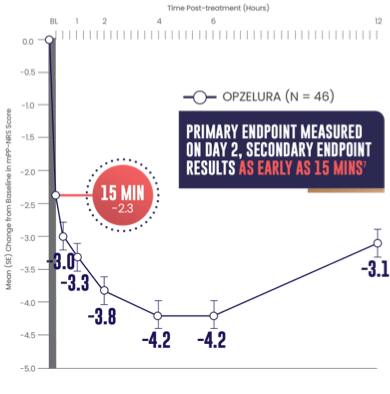
- Primary endpoint: mean change from baseline PP-NRS (6.7) on Day 2 was -3.47
- Secondary endpoint: 15 minutes after the first OPZELURA application the mean change from baseline in mPP-NRS was -2.37
- Data were reported as observed7
- No conclusions of safety or efficacy should be made based on these results
Adapted from Bissonnette et al. Revolutionizing Atopic Dermatitis. 2023.
The line graph above shows that OPZELURA (n = 46) demonstrated a mean change from baseline in mPP-NRS score of -2.3 at 15 minutes. At 30 minutes, the mean change was -3.0. At 1 hour, the mean change was -3.3. At 2 hours, the mean change was -3.8. At 4 and 6 hours, the mean change was -4.2. At 12 hours post treatment, the mean change was -3.1.5,7
Secondary endpoints included: change from baseline in mPP-NRS at 15 and 30 minutes and at 1, 2, 4, 6, and 12 hours post-treatment on Day 1, change from baseline in PP-NRS on Day 3 through Day 29, and change from baseline in IGA at Days 8, 15, and 29.7
TEAEs were reported in 30.6% of participants; all were grade 1 or 2; none were serious.7
AD, atopic dermatitis; BL, baseline; IGA, Investigator’s Global Assessment; min, minutes; mPP-NRS, modified peak pruritus numerical rating scale;
PP-NRS, peak pruritus numerical rating scale (PP-NRS is reported as the worst level of itch during the past 24-hour period from 0 [no itch] to 10 [worst imaginable itch]); SE, standard error; TEAE, treatment-emergent adverse event.


