skin clearance results
make a clear difference with opzelura1


PRIMARY ENDPOINT
CLEAR OR ALMOST CLEAR RESULTS (IGA-TS*) AT WEEK 81-3


MORE THAN 1 IN 2 PATIENTS ACHIEVED CLEAR OR ALMOST CLEAR SKIN WITH ≥2-POINT IMPROVEMENT (IGA-TS) FROM BASELINE AT WEEK 81-3
*IGA-TS is defined as the achievement of clear (IGA 0) or almost clear (IGA 1) skin with at least a 2-point improvement from baseline; IGA is assessed on a severity scale of 0 to 4.1
BID, twice daily; IGA, Investigator’s Global Assessment; IGA-TS, Investigator’s Global Assessment treatment success.
Adapted from Papp K et al. doi:10.17632/ffx6nd5zyb.1. Licensed under CC BY 4.0
The line graph above shows the proportion of IGA-TS responders for OPZELURA and vehicle from the TRuE-AD1 and TRuE-AD2 clinical trials from Week 0 to Week 8. At Week 8, 53.8% of patients taking OPZELURA achieved clear or almost clear results vs. 15.1% for vehicle in TRuE-AD1. In TRuE-AD2, 51.3% of patients taking OPZELURA achieved clear or almost clear results vs. 7.6% for vehicle. This demonstrates over 3x as many responders to OPZELURA than vehicle.1-3
EASI RESULTS
DELIVERED MEANINGFUL IMPROVEMENT IN EASI SCORES2,3
≥75% IMPROVEMENT IN LESION EXTENT AND SEVERITY (EASI-75*) AT WEEK 82,3


>60% of patients achieved EASI-75 at Week 82
In TRuE-AD1 and TRuE-AD2, respectively, 46% and 50% of patients on OPZELURA vs. 56% and 44% of patients on vehicle had a mild EASI score (defined as between 1.1 to 7.0) at baseline.4,5
*EASI-75 is defined as the achievement of at least 75% improvement in EASI score from baseline.2
BID, twice daily; EASI, Eczema Area and Severity Index.
Adapted from Papp K et al. doi:10.17632/ffx6nd5zyb.1. Licensed under CC BY 4.0
The line graph above shows the proportion of patients achieving EASI-90 at Week 8. In TRuE-AD1 and TRuE-AD2, respectively, 62.1% and 61.8% of patients on OPZELURA vs. 24.6% and 14.4% of patients on vehicle achieved EASI-75 at Week 8.2,3
≥90% IMPROVEMENT IN LESION EXTENT AND SEVERITY (EASI-90*) AT WEEK 82,3


>40% of patients achieved EASI-90 at Week 82
In TRuE-AD1 and TRuE-AD2, respectively, 46% and 50% of patients on OPZELURA vs. 56% and 44% of patients on vehicle had a mild EASI score (defined as between 1.1 to 7.0) at baseline.4,5
*EASI-90 is defined as the achievement of at least 90% improvement in EASI score from baseline.2
BID, twice daily; EASI, Eczema Area and Severity Index.
Adapted from Papp K et al. doi:10.17632/ffx6nd5zyb.1. Licensed under CC BY 4.0
The line graph above shows the proportion of patients achieving EASI-90 at Week 8. In TRuE-AD1 and TRuE-AD2, respectively, 44.3% and 43.4% of patients on OPZELURA vs. 9.5% and 4.2% of patients on vehicle achieved EASI-90 at Week 8.2,3
WHAT ARE PATIENTS SAYING ABOUT OPZELURA?
LONG-TERM DISEASE CONTROL (EXTENSION DATA)
SUSTAINED DISEASE CONTROL THROUGH 52 WEEKS WITH AS-NEEDED USE OF MONOTHERAPY6
PATIENTS ACHIEVING IGA* SCORES OF 0/1 THROUGH WEEK 52 (EXTENSION DATA)6


*IGA is assessed on a severity scale of 0 to 4.1
>70% OF PATIENTS HAD CLEAR OR ALMOST CLEAR SKIN WITH AS-NEEDED USE OF OPZELURA AT WEEK 526
- More patients achieved an IGA score of 0 or 1 after switching from vehicle to OPZELURA in the 44-week extension period6
- Final proportions based on number of remaining patients
- Data were reported as observed6
- No conclusions of safety or efficacy should be made based on these results
- OPZELURA is for short-term and non-continuous use only1
The line graph above shows the proportion of patients achieving IGA scores of 0/1 with OPZELURA at Week 52. In TRuE-AD1 and TRuE-AD2, respectively, 75.4% and 80.1% of patients on OPZELURA vs. 73.7% and 74.4% of patients on vehicle achieved clear or almost clear skin after switching from vehicle to OPZELURA and using as needed in the 44-week extension period. 80% to 90% of patients maintained or improved response between subsequent visits.6
BID, twice daily; BSA, body surface area; IGA, Investigator's Global Assessment.
BID, twice daily; BSA, body surface area; IGA, Investigator's Global Assessment.

Patients spent 44% of the 44-Week LTS periods between weeks 8 and 52 off treatment due to lesion clearance.7,8
Data were reported as observed.7,8
No conclusions of safety or efficacy should be made based on these results.

BSA REDUCTION THROUGH 1 YEAR4,8


LTS data were reported as observed. No conclusions of safety or efficacy should be made based on these results.
OPZELURA is for short-term and non-continuous use only.1
*Among patients who continued into the LTS period.
≈70% reduction in mean BSA after 8 weeks of OPZELURA use, from 9.6% to 2.9% (from 9.3% to 2.8% in TRuE-AD1 and from 9.5% to 3.0% in TRuE-AD2).4
≈3X reduction at 8 weeks with OPZELURA vs. vehicle. Affected BSA maintained with as-needed treatment with OPZELURA through 52 weeks.4,8
The visualization above shows the reduction in mean total affected BSA from the pooled LTS data from TRuE-AD1 and TRuE-AD2 clinical trials from baseline to Week 52. Patients taking OPZELURA experienced a reduction to 1.4% mean total affected BSA by Week 52 vs. a reduction to 1.7% for those taking the vehicle-to-OPZELURA.8
AD, atopic dermatitis; BID, twice daily; BSA, body surface area; LTS, long-term double-blind safety; VC, vehicle-controlled.
SELECT ENDPOINT TO SEE VISUALIZATION

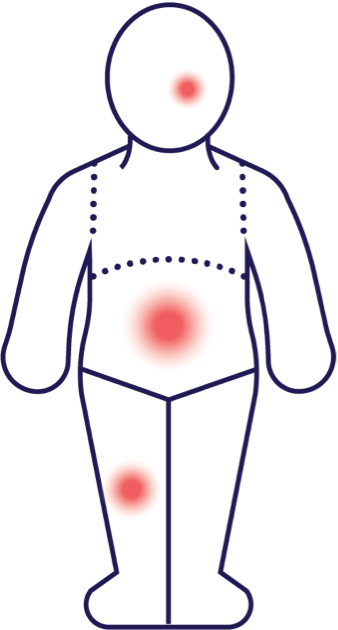


MEAN TOTAL AFFECTED BSA (%)
OPZELURA Vehicle-to-OPZELURA
LTS data were reported as observed.4,8
No conclusions of safety or efficacy should be made based on these results.
This figure is intended to illustrate the mean affected BSA reduction in the OPZELURA arm (pooled LTS data from TRuE-AD1 and TRuE-AD2) using hypothetical lesions, not necessarily depicting actual results. However, the size of the lesions has been mathematically calculated to ensure accuracy relative to each body region. Results may vary.4,8
For topical use only. Not for ophthalmic, oral, or intravaginal use. Instruct patients to apply a thin layer of OPZELURA twice daily to affected areas of up to 20% BSA. Patients should stop using when signs and symptoms of AD resolve. If signs and symptoms do not improve within 8 weeks, patients should be re-examined by their HCP.1
≈70% reduction in mean BSA after 8 weeks of OPZELURA use, from 9.6% to 2.9% (from 9.3% to 2.8% in TRuE-AD1 and from 9.5% to 3.0% in TRuE-AD2).4
≈3X reduction at 8 weeks with OPZELURA vs. vehicle. Affected BSA maintained with as-needed treatment with OPZELURA through 52 weeks.4,8
The visualization above shows the reduction in mean total affected BSA from the pooled LTS data from TRuE-AD1 and TRuE-AD2 clinical trials from baseline to Week 52. Patients taking OPZELURA experienced a reduction to 1.4% mean total affected BSA by Week 52 vs. a reduction to 1.7% for those taking the vehicle-to-OPZELURA.8
AD, atopic dermatitis; BID, twice daily; BSA, body surface area; LTS, long-term double-blind safety; VC, vehicle-controlled.
PHASE 2 DOSE-RANGING STUDY
OPZELURA, TRIAMCINOLONE CREAM 0.1%, AND VEHICLE9,10
PHASE 2, RANDOMIZED, DOUBLE-BLIND, DOSE-RANGING, VEHICLE- AND ACTIVE-CONTROLLED STUDY IN ADULT PATIENTS WITH AD9,10


PRIMARY ENDPOINT RESULTS:
MEAN CFB IN EASI SCORE AT WEEK 4 (OPZELURA: 71.6%, vehicle: 15.5%; P < 0.0001).9
No statistical comparison between triamcinolone and OPZELURA is shown.9,10
Enrollment criteria: aged 18-70, history of AD for ≥2 years, IGA score of 2 or 3, affected BSA of 3% to 20%.9
Following the 8-week, double-blind period, patients in all treatment arms were treated with OPZELURA BID for an additional 4 weeks (open-label period), followed by a 4-week follow-up period.9,10
Treatment of facial dermatitis was not permitted because of the restrictions on the use of triamcinolone on the face.9
The graphic above shows the study design for the phase 2, randomized, double-blind, dose-ranging, vehicle- and active-controlled study in adult patients with AD. 307 patients were randomized evenly for 8 weeks of double-blind treatment: vehicle, triamcinolone cream (0.1% BID for 4 weeks, then vehicle for 4 weeks), OPZELURA BID, ruxolitinib cream 1.5% QD, ruxolitinib cream 0.5% QD, or ruxolitinib cream 0.15% QD.9
SELECT SECONDARY ENDPOINTS




Descriptive analysis; no statistical comparison between triamcinolone and OPZELURA is shown.9,10
No conclusions of efficacy and safety should be made based on these results.
The bar graph shows the comparison of the mean percentage improvement from baseline in EASI score at Week 4, with OPZELURA (n = 50) demonstrating a 71.6% mean CFB, vehicle (n = 52) a 15.5% mean CFB, and triamcinolone (n = 51) a 59.8% mean CFB.9
The bar graph shows the comparison of the proportion of patients achieving Itch NRS4 at Week 4, with OPZELURA (n = 40) demonstrating 62.5% responders, vehicle (n = 36) 11.1% responders, and triamcinolone (n = 11) 32.2% responders.10
In the 8-week double-blind period, TEAEs were reported in 24% (n = 12) of OPZELURA BID patients and 33.3% (n = 17), of TRIAM BID patients; no serious TEAEs related to the study drug occurred. Most common TEAEs occurring in >1% of patient population were nasopharyngitis (OPZELURA BID 4%, TRIAM BID 0%), AD (0%, 3.9%), upper respiratory tract infection (2%, 2%), application site pain (2%, 0%), headache (4%, 0%), and urinary tract infection (0%, 2%).9
*Mean CFB in EASI is defined as mean percent change in lesion extent and severity from baseline at Week 4; patients in the analysis had an EASI score of 8.4 ± 4.7 at baseline.9
†Itch NRS4 is defined as the achievement of at least a 4-point improvement in daily itch on a 0- to 10-point scale, considered a clinically meaningful response. Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,2
AD, atopic dermatitis; BID, twice daily; BSA, body surface area; CFB, change from baseline; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, numerical rating scale; QD, once daily; TEAE, treatment-emergent adverse event; TRIAM, triamcinolone.


