PATIENT PHOTOS
SEE HOW OPZELURA MAY BE ABLE TO HELP YOUR PATIENTS



CONSIDER OPZELURA FOR ALL YOUR APPROPRIATE PATIENTS WITH ITCHY, MILD TO MODERATE AD1
A patient for whom OPZELURA may be appropriate1:
- Has itchy, mild to moderate atopic dermatitis
- Is 12 years of age or older
- Has an affected BSA of up to 20%
- Has tried topical corticosteroid (TCS) and/or topical calcineurin inhibitor (TCI), unless the use of such alternatives is not advisable
- Will not be using OPZELURA in combination with:
- Therapeutic biologics
- Other JAK inhibitors
- Potent immunosuppressants
In particular, OPZELURA may be an appropriate choice for patients for whom other topicals haven’t worked, but who do not want to move onto an injectable or systemic treatment.1
Routine bloodwork when using OPZELURA is not required. However, blood tests may be called for to evaluate potential side effects or for patients with a history of hematologic malignancies.1
BSA, body surface area; JAK, Janus kinase.
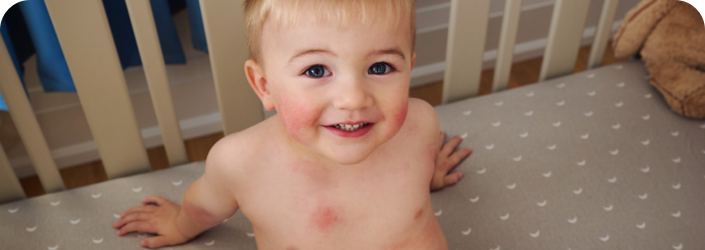
CLINICAL TRIAL PHOTOS
SEE THE OPZELURA DIFFERENCE
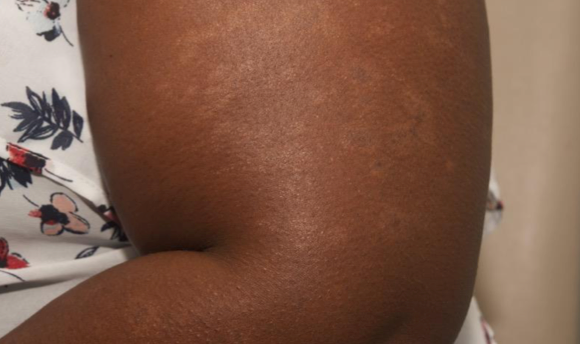
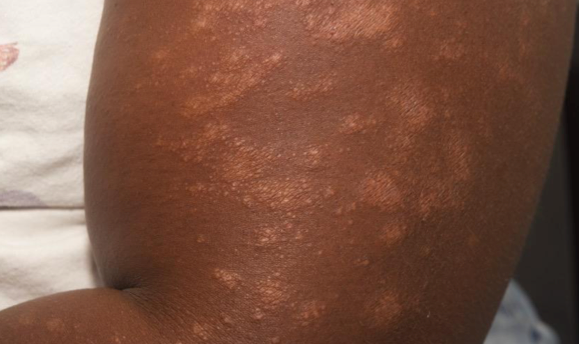
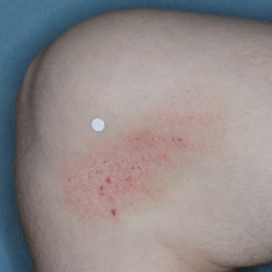
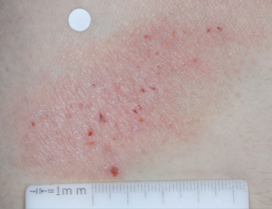
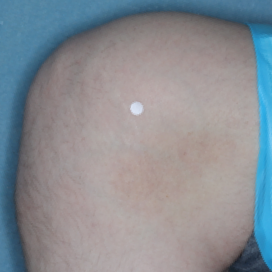
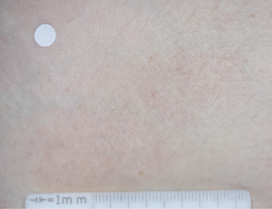
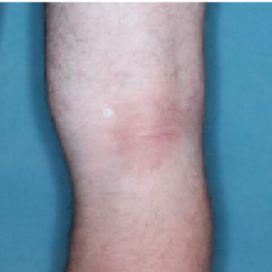
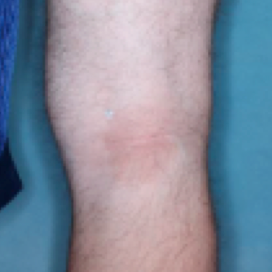
PATIENT 1 - KNEE2
BASELINE
(IGA* = 3, NRS† = 3.57, BSA = 3%)
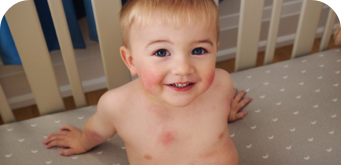
WEEK 2
(IGA = 1, NRS = 0.43, BSA = 2%)
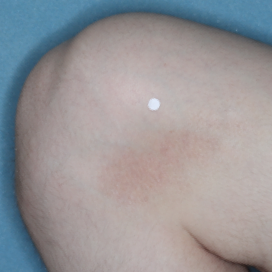
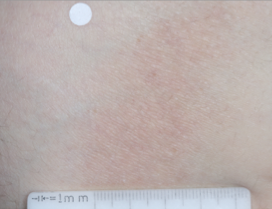
WEEK 8
(IGA = 0, NRS = 0, BSA = 0%)
Actual clinical trial participant. Individual results may vary.
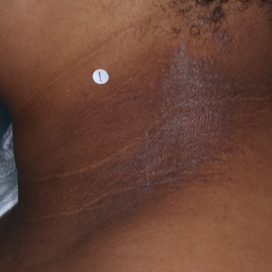
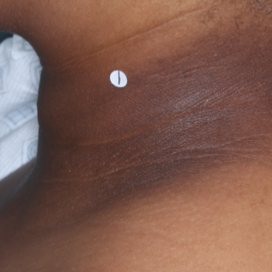
PATIENT 2 - NECK2
BASELINE
(IGA* = 3, NRS† = 8.57, BSA = 5.5%)
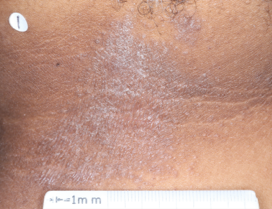
WEEK 2
(IGA = 2, NRS = 1.75, BSA = 4.5%)
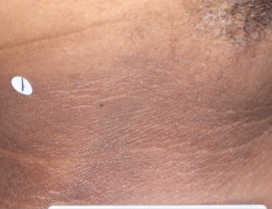
WEEK 8
(IGA = 1, NRS = N/A, BSA = 0.1%)
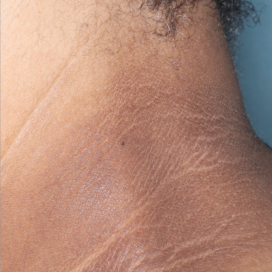

Actual clinical trial participant. Individual results may vary.
PATIENT 3 - BACK OF KNEE2
BASELINE
(IGA* = 3, NRS† = 3.86, BSA = 7%)
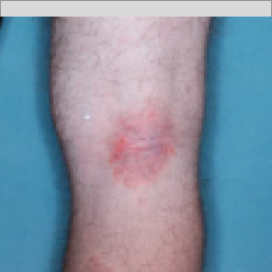
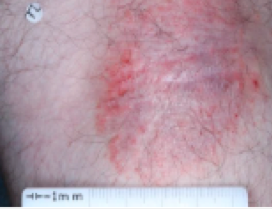
WEEK 2
(IGA = 2, NRS = 0, BSA = 3%)
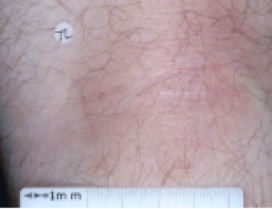
WEEK 8
(IGA = 1, NRS = 0, BSA = 1%)
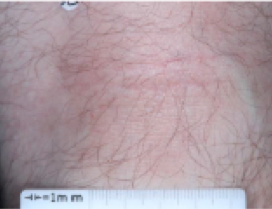
Actual clinical trial participant. Individual results may vary.
PATIENT 4 - CALF2
BASELINE
(IGA* = 3, NRS† = 5.86, BSA = 3.5%)
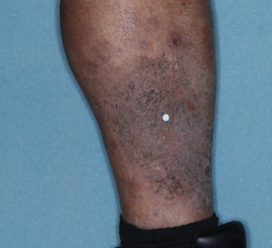

WEEK 2
(IGA = 2, NRS = 1.57, BSA = 1.75%)
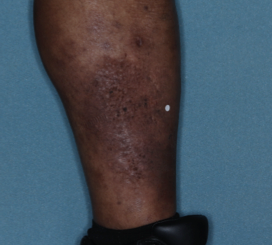
WEEK 8
(IGA = 0, NRS = 0, BSA = 0%)
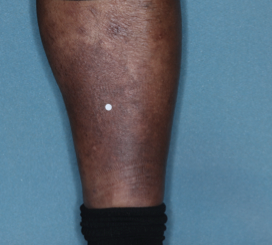
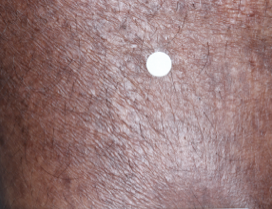
Actual clinical trial participant. Individual results may vary.
PATIENT 5 - BACK2
BASELINE
(IGA* = 3, NRS† = 7.57, BSA = 17%)
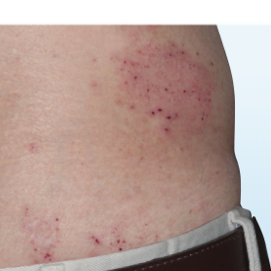
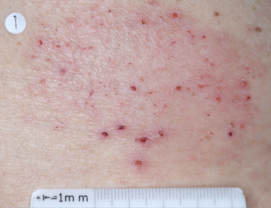
WEEK 2
(IGA = 1, NRS = 3, BSA = 4.5%)
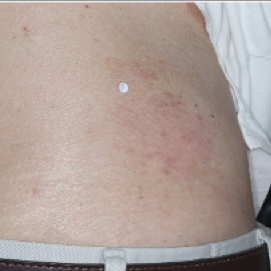
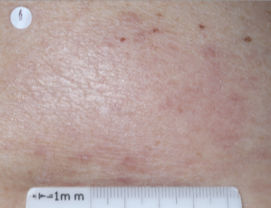
WEEK 8
(IGA = 1, NRS = 0, BSA = 0.1%)
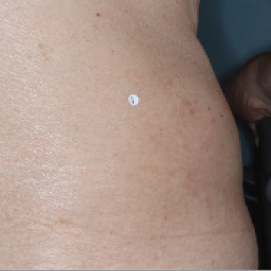
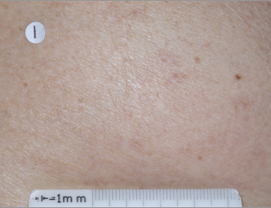
Actual clinical trial participant. Individual results may vary.
PATIENT 6 - NECK2
BASELINE
(IGA* = 3, NRS† = 2.43, BSA = 8.2%)
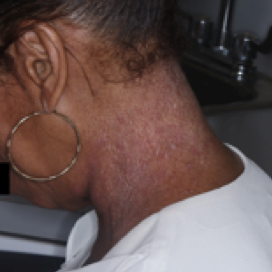
WEEK 2
(IGA = 2, NRS = 1.43, BSA = 5.6%)
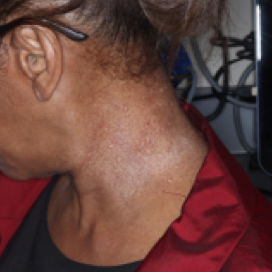
WEEK 8
(IGA = 1, NRS = 2.43, BSA = 2.3%)
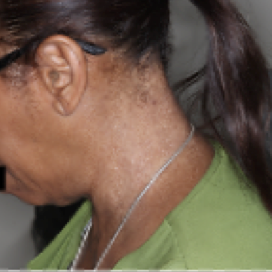
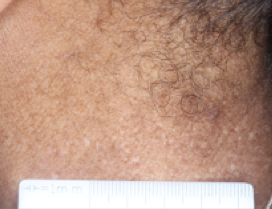
Actual clinical trial participant. Individual results may vary.
*Measured by IGA-TS, defined as the achievement of clear (IGA 0) or almost clear (IGA 1) skin with at least a 2-point improvement from baseline; IGA is assessed on a severity scale of 0 to 4.1
†Itch NRS4 is defined as the achievement of at least a 4-point improvement in daily itch on a 0- to 10-point scale, considered a clinically meaningful response.
Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,3
BSA, body surface area; IGA, Investigator's Global Assessment; IGA-TS, Investigator's Global Assessment treatment success; NRS, numerical rating scale.
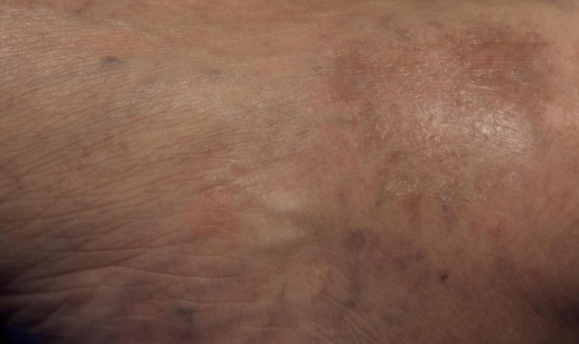
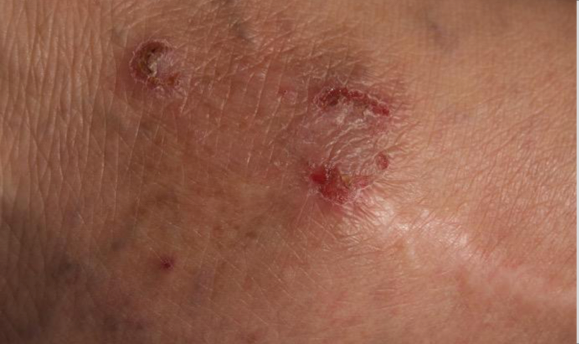
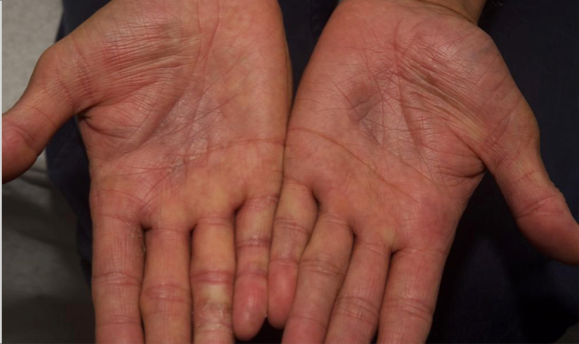
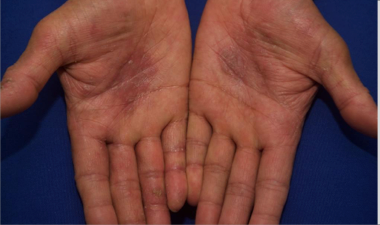
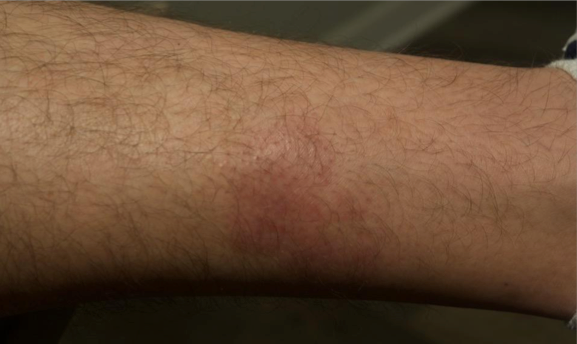
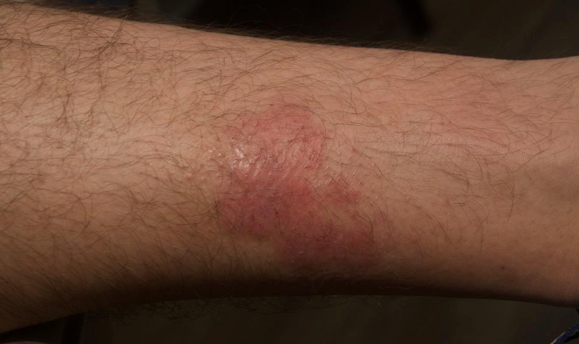
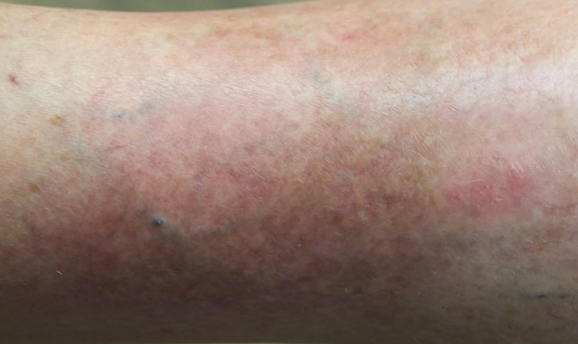
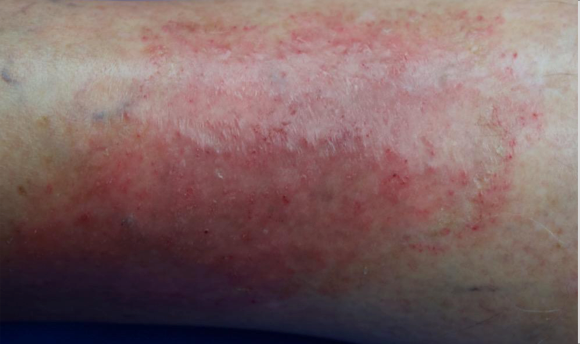
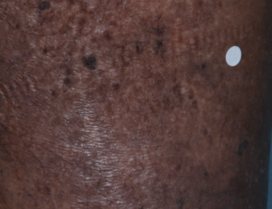
Visualizing real-world results with Opzelura2
The following images depict actual patients treated with OPZELURA. Not clinical trial participants. Scoring was designated by the treating physician. Because the following patients are real-world patients, there may be other factors influencing their treatment results, and individual results may vary.
Actual patient treated with OPZELURA. Not a clinical trial participant.
Scoring was designated by the treating physician. Because this is a real-world patient, there may be other factors influencing treatment results, and individual results may vary. No conclusions of safety or efficacy should be made based on these results.
Photos by Dr. Jason Smith, MD (Dermatologist - Rome, GA).
BSA, body surface area; IGA, Investigator's Global Assessment.
“TALK SOONER"
An online quantitative survey* was conducted on behalf of Incyte from March 19 to May 10, 2024 including 95 current OPZELURA users with AD.2
According to the 95 AD patients,
96% OF PATIENTS WISHED THEY KNEW ABOUT OPZELURA SOONER2
Data were self-reported and outcomes may vary. No conclusions of safety or efficacy should be made based on these results.
Limitations: This survey relied on self-reported data, was not confirmed by a medical professional, and can be affected by bias from selection, recall, response styles, and other sources. Opinions may not be representative of the general population.

*Participants with AD reported they: had received an AD diagnosis; had been using OPZELURA for ≥2 weeks; were current or former users of topical corticosteroids and/or non-steroidal creams.2
The survey covered condition and treatment background, AD disease state, OPZELURA experience, and overall OPZELURA satisfaction.2
AD, atopic dermatitis.