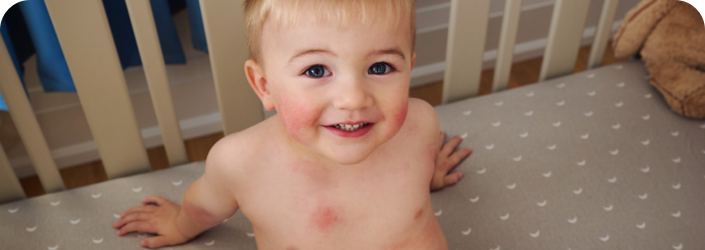
SAVINGS
DISCOVER THE OPZELURA COPAY SAVINGS CARD
THE COPAY SAVINGS CARD IS READY TO DOWNLOAD
Copay Savings Card for OPZELURA Terms and Conditions
By using the copay savings card for OPZELURA, the patient and, if applicable, the healthcare provider and/or pharmacist, acknowledges that the patient meets the eligibility criteria and understands the Terms and Conditions described below:
- The patient is not eligible to use this copay savings card if they are enrolled in a state or federally funded prescription insurance program, including, but not limited to, Medicare, Medicaid, TRICARE, Veterans Affairs health care, a state prescription drug assistance program, or the Government Health Insurance Plan available in Puerto Rico (formerly known as “La Reforma de Salud”)
- The patient must have commercial insurance. Offer is not valid if the patient is uninsured, paying cash for the prescription, or is covered by an Alternate Funding Program (AFP)
- By using this copay savings card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for OPZELURA may pay as little as $0 per tube. Eligible patients whose commercial insurance plan does not cover OPZELURA will pay $35 per prescription and will only be able to redeem this offer at certain participating pharmacies. Other offers may be available for these patients at other pharmacies
- Individual out-of-pocket cost may vary based on the price at the pharmacy
- Maximum benefits per tube and per calendar year apply. For more information, call 833-613-2333
- Patient enrollment in a copay adjustment program, such as a maximizer or accumulator program, may impact the value of this offer. Incyte will monitor program utilization data and reserves the right to vary or discontinue program benefits at any time if Incyte determines that patients are subject to a copay maximizer or copay accumulator program
- This copay savings card is not valid when the entire cost of the patient’s prescription drug is eligible to be reimbursed by their commercial insurance plan or any other health or pharmacy benefit program
- Neither the patient, nor the patient’s guardian, pharmacist, or doctor may seek any third-party reimbursement for the value of the copay savings received under this offer, including any health insurance program or plan, flexible spending account, or health care savings account
- The patient is responsible for reporting use of the copay savings card to any commercial insurer, health plan, or other third party that pays for or reimburses any part of the prescription filled using the copay savings card, as may be required. The patient should not use the copay savings card if their insurer or health plan prohibits use of manufacturer copay cards
- This copay savings card is not valid where prohibited by law. The $35 offer is not valid for Massachusetts patients whose commercial insurance does not cover OPZELURA
- This copay savings card cannot be combined with any other savings, free trial, or similar offer for the specified prescription
- This copay savings card will be accepted only at participating pharmacies
- This copay savings card is not health insurance
- Offer good only in the U.S. and Puerto Rico
- The copay savings card benefit may not be redeemed more than once per 25 days per patient
- Offer valid only for an FDA-approved use
- No other purchase is necessary
- Data related to the patient’s redemption of the copay savings card may be collected, analyzed, and shared with Incyte or its affiliates for market research and other purposes related to assessing Incyte’s programs
- By enrolling in this copay savings program, the patient acknowledges that Incyte may use their information and share it with providers or their insurance plan in connection with providing copay savings support and for the other purposes related to the copay savings program. Incyte may also share the patient’s information with its subsidiaries, affiliates, representatives, agents, and contractors. Incyte may also de-identify the patient’s information and use the de-identified information for Incyte’s business purposes
Incyte reserves the right to rescind, revoke, or amend this offer at any time without notice.
For questions or additional support call 833-613-2333 or email [email protected].