TRIAL OVERVIEW: AGES 2-11
EVALUATED IN A PHASE 3 TRIAL OF PEDIATRIC PATIENTS1-3


330 pediatric patients 2-11 years of age were included in a phase 3, double-blind, randomized, vehicle-controlled trial.1-3
Patients were randomized to monotherapy with OPZELURA, ruxolitinib cream 0.75%, or vehicle BID for 8 weeks and as needed during a 44-week extension.1-3
key inclusion criteria2,3:
- 2-11 years of age
- Diagnosis of AD ≥3 months
- IGA score of 2 or 3*
- Affected BSA of 3% to 20%
- Itch NRS ≥4 (among patients aged 6-11 years)
| Primary endpoint3 | |
| IGA-TS | Proportion of patients who achieved clear (IGA 0) or almost clear (IGA 1) skin with at least a 2-point improvement from baseline |
Safety and tolerability assessments included the frequency of reported TEAEs, treatment-related AEs, and AEs leading to discontinuation.2,3
*Severity scale of 0 to 4.1

At baseline:
- Patients had a mean affected BSA of ≈11%3
- 76% of the patients had an IGA score of 3 (moderate)3
AD, atopic dermatitis; AE, adverse event; BSA, body surface area; IGA, Investigator's Global Assessment; IGA-TS, Investigator’s Global Assessment treatment success; NRS, numerical rating scale; TEAE, treatment-emergent adverse event.
BASELINE PATIENT CHARACTERISTICS1,3
| Study Participants (N = 330) | |
|---|---|
| Age (years) | |
| 2-6 | 51% |
| 7-<12 | 49% |
| Race | |
| White | 55% |
| Black | 32% |
| Asian | 6% |
| Other | 7% |
| Lesion appearance (IGA*) | |
| Score of 2 | 24% |
| Score of 3 | 76% |
| BSA % | |
| Mean | 10.5% |
*Patients had a baseline IGA score of 2 to 3 on a severity scale of 0 to 4.1
BSA, body surface area; IGA, Investigator's Global Assessment.
- Patients initially randomized to OPZELURA in the TRuE-AD3 clinical trial remained on their regimen2
- Patients initially randomized to vehicle were rerandomized 1:1 to ruxolitinib cream 0.75% or OPZELURA2
- Patients self-evaluated active AD lesions and treated as needed (up to 20% BSA)2
- Patients were instructed to stop treatment 3 days after lesion clearance and restart treatment at the first sign of recurrence2
- If new lesions were extensive or appeared in new areas, patients were instructed to contact the investigator to determine if an unscheduled additional visit was needed. Rescue treatment was not permitted2
- Study visits occurred every 4 weeks2
- Patients initially randomized to OPZELURA in the TRuE-AD3 clinical trial remained on their regimen2
- Patients initially randomized to vehicle were rerandomized 1:1 to ruxolitinib cream 0.75% or OPZELURA2
- Patients self-evaluated active AD lesions and treated as needed (up to 20% BSA)2
- Patients were instructed to stop treatment 3 days after lesion clearance and restart treatment at the first sign of recurrence2
- If new lesions were extensive or appeared in new areas, patients were instructed to contact the investigator to determine if an unscheduled additional visit was needed. Rescue treatment was not permitted2
- Study visits occurred every 4 weeks2
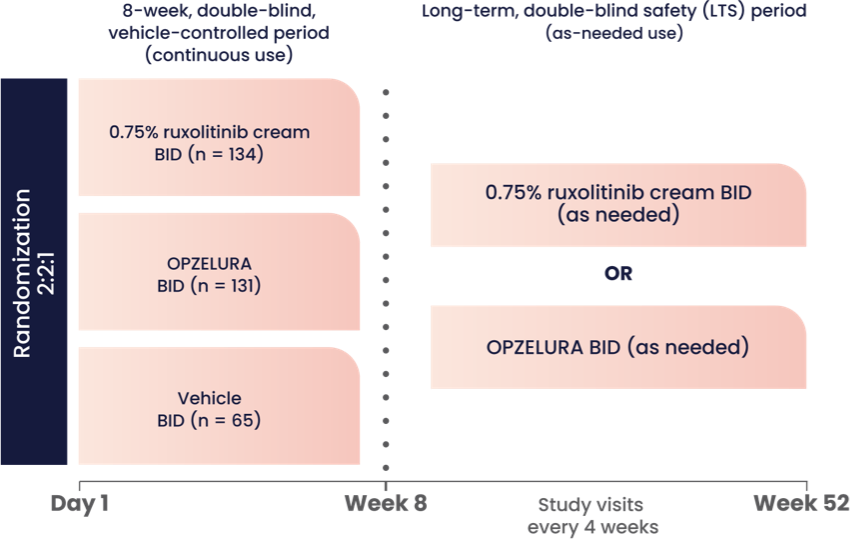
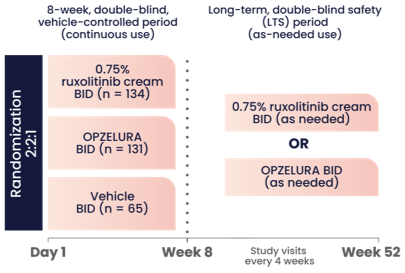
The graphic above shows the study design for OPZELURA, which was studied in a double-blind, randomized, vehicle-controlled trial (TRuE-AD3). The study included 330 pediatric patients 2-11 years of age with an affected BSA of 3% to 20% and IGA score of 2 (24% of subjects) to 3 (76% of subjects) on a severity scale of 0 to 4. Patients were randomized to monotherapy with OPZELURA, ruxolitinib cream 0.75%, or vehicle twice daily for 8 weeks.1
Eligible patients could continue treatment for an additional 44 weeks in an extension period where OPZELURA was applied to AD areas twice daily as needed. Patients were instructed to stop treatment 3 days after lesion clearance and restart at the first sign of recurrence.1,2
AD, atopic dermatitis; BID, twice daily; BSA, body surface area; IGA, Investigator's Global Assessment.


